Since Kr or Krypton is a noble gas, it has a full valence shell or octet of 8 electrons. If this helped, leave a like:). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of krypton (atomic number: 36), the most common. Box spin diagram of outer electron orbitals for the electron configuration of the atom. 36 Krypton, Kr, Ar3ds24p6 = Kr , Ar3d 4s 4p v. 2021: Valence Electrons in Krypton (Kr) & Facts, Color, Discovery. A valence electron is an outer shell electron and may participate in the formation of a chemical bond. Ok but how many valence electrons does an atom of Krypton have? In the case of Krypton the valence electrons is 0. Naturally occurring krypton in Earth's atmosphere is composed of five stable isotopes, plus one isotope (78 Kr) with such a long half-life (9.2×10 21 years) that it can be considered stable. (This isotope has the second-longest known half-life among all isotopes for which decay has been observed; it undergoes double electron capture to 78 Se). Give the correct number of valence electrons for the element krypton, Kr, atomic #36. Check Give the correct number of valence electrons for the element gallium, Ga, atomic #31.
What is the ground-state electron configuration of the element Kr?
1 Answer
OR
OR
Explanation:
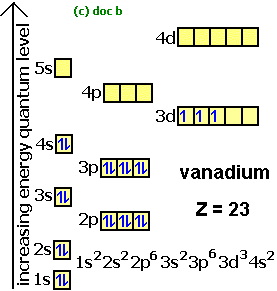
First: Determine the number of electrons. Krypton has a total of 36 electrons
Second: KNOW YOUR ORBITALS
Know how many electrons each orbital can hold and their order.
(Refer to the following pictures as notes)
Third: Write out the electron configuration
For Krypton and most of the elements there are more the just one way (usually two) to write the electron configuration. One way is to write out the entire electron configuration by going through each orbital or we can use a shorthand notation using the noble gases as a starting point. I will go through both methods:
First Method: (Long way)
We know that Krypton has
We know that the
Second Method (Shorthand)
The key to using this method is to identify the noble gas closest to the desired element that is at a lower energy (Has a lower atomic number if I'm loosely speaking). In essence, the shorthand notation tells us the configuration by using a noble gas element as our starting point instead of starting all the way at the
Coincidently, Krypton itself is a noble gas so we could write the electron configuration as
*Notice how the configuration
All in all, the three given answers are correct ways of figuring out the ground-state electron configuration of Krypton .
Related questions
Quantum Numbers,
Atomic Orbitals, and
Electron Configurations
Contents:
Quantum Numbers and Atomic Orbitals
1. Principal Quantum Number (n)
2.Angular Momentum (Secondary, Azimunthal) Quantum Number (l)
3.Magnetic Quantum Number (ml)
4.Spin Quantum Number (ms)
Table of Allowed Quantum Numbers
Writing Electron Configurations
Properties of Monatomic Ions
References
Quantum Numbers and Atomic Orbitals
By solving the Schrödinger equation (Hy = Ey), we obtain a set of mathematical equations, called wave functions (y), which describe the probability of finding electrons at certain energy levels within an atom.
A wave function for an electron in an atom is called an atomic orbital; this atomic orbital describes a region of space in which there is a high probability of finding the electron. Energy changes within an atom are the result of an electron changing from a wave pattern with one energy to a wave pattern with a different energy (usually accompanied by the absorption or emission of a photon of light).
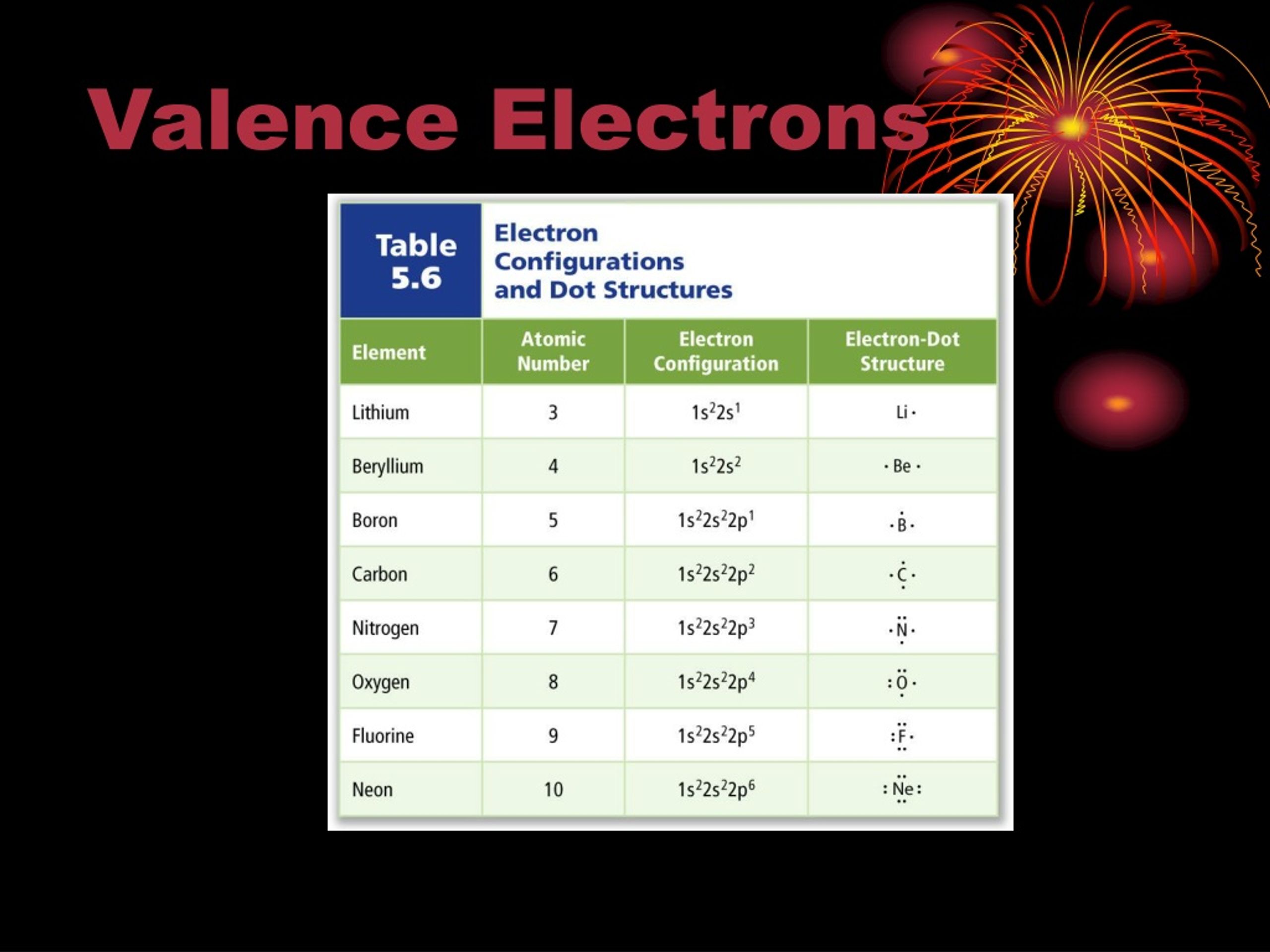
Each electron in an atom is described by four different quantum numbers. The first three (n, l, ml) specify the particular orbital of interest, and the fourth (ms) specifies how many electrons can occupy that orbital.
- Principal Quantum Number (n): n = 1, 2, 3, …, ∞
Specifies the energy of an electron and the size of the orbital (the distance from the nucleus of the peak in a radial probability distribution plot). All orbitals that have the same value of n are said to be in the same shell (level). For a hydrogen atom with n=1, the electron is in its ground state; if the electron is in the n=2 orbital, it is in an excited state. The total number of orbitals for a given n value is n2.

- Angular Momentum (Secondary, Azimunthal) Quantum Number (l): l = 0, ..., n-1.
Specifies the shape of an orbital with a particular principal quantum number. The secondary quantum number divides the shells into smaller groups of orbitals called subshells (sublevels). Usually, a letter code is used to identify l to avoid confusion with n:
| l | 0 | 1 | 2 | 3 | 4 | 5 | ... |
| Letter | s | p | d | f | g | h | ... |
How Many Valence Electrons Does Kr Have
The subshell with n=2 and l=1 is the 2p subshell; if n=3 and l=0, it is the 3s subshell, and so on. The value of l also has a slight effect on the energy of the subshell; the energy of the subshell increases with l (s < p < d < f).
- Magnetic Quantum Number (ml): ml = -l, ..., 0, ..., +l.
Specifies the orientation in space of an orbital of a given energy (n) and shape (l). This number divides the subshell into individual orbitals which hold the electrons; there are 2l+1 orbitals in each subshell. Thus the s subshell has only one orbital, the p subshell has three orbitals, and so on.
- Spin Quantum Number (ms): ms = +½ or -½.
Specifies the orientation of the spin axis of an electron. An electron can spin in only one of two directions (sometimes called up and down).
The Pauli exclusion principle (Wolfgang Pauli, Nobel Prize 1945) states that no two electrons in the same atom can have identical values for all four of their quantum numbers. What this means is that no more than two electrons can occupy the same orbital, and that two electrons in the same orbital must have opposite spins.
Because an electron spins, it creates a magnetic field, which can be oriented in one of two directions. For two electrons in the same orbital, the spins must be opposite to each other; the spins are said to be paired. These substances are not attracted to magnets and are said to be diamagnetic. Atoms with more electrons that spin in one direction than another contain unpaired electrons. These substances are weakly attracted to magnets and are said to be paramagnetic.
Table of Allowed Quantum Numbers
| n | l | ml | Number of orbitals | Orbital Name | Number of electrons |
| 1 | 0 | 0 | 1 | 1s | 2 |
| 2 | 0 | 0 | 1 | 2s | 2 |
| 1 | -1, 0, +1 | 3 | 2p | 6 | |
| 3 | 0 | 0 | 1 | 3s | 2 |
| 1 | -1, 0, +1 | 3 | 3p | 6 | |
| 2 | -2, -1, 0, +1, +2 | 5 | 3d | 10 | |
| 4 | 0 | 0 | 1 | 4s | 2 |
| 1 | -1, 0, +1 | 3 | 4p | 6 | |
| 2 | -2, -1, 0, +1, +2 | 5 | 4d | 10 | |
| 3 | -3, -2, -1, 0, +1, +2, +3 | 7 | 4f | 14 |
Writing Electron Configurations
The distribution of electrons among the orbitals of an atom is called the electron configuration. The electrons are filled in according to a scheme known as the Aufbau principle ('building-up'), which corresponds (for the most part) to increasing energy of the subshells:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f
It is not necessary to memorize this listing, because the order in which the electrons are filled in can be read from the periodic table in the following fashion:
Or, to summarize:
In electron configurations, write in the orbitals that are occupied by electrons, followed by a superscript to indicate how many electrons are in the set of orbitals (e.g., H 1s1)
Another way to indicate the placement of electrons is an orbital diagram, in which each orbital is represented by a square (or circle), and the electrons as arrows pointing up or down (indicating the electron spin). When electrons are placed in a set of orbitals of equal energy, they are spread out as much as possible to give as few paired electrons as possible (Hund's rule).
examples will be added at a later date
In a ground state configuration, all of the electrons are in as low an energy level as it is possible for them to be. When an electron absorbs energy, it occupies a higher energy orbital, and is said to be in an excited state.
Properties of Monatomic Ions
The electrons in the outermost shell (the ones with the highest value of n) are the most energetic, and are the ones which are exposed to other atoms. This shell is known as the valence shell. The inner, core electrons (inner shell) do not usually play a role in chemical bonding.
Elements with similar properties generally have similar outer shell configurations. For instance, we already know that the alkali metals (Group I) always form ions with a +1 charge; the 'extra' s1 electron is the one that's lost:

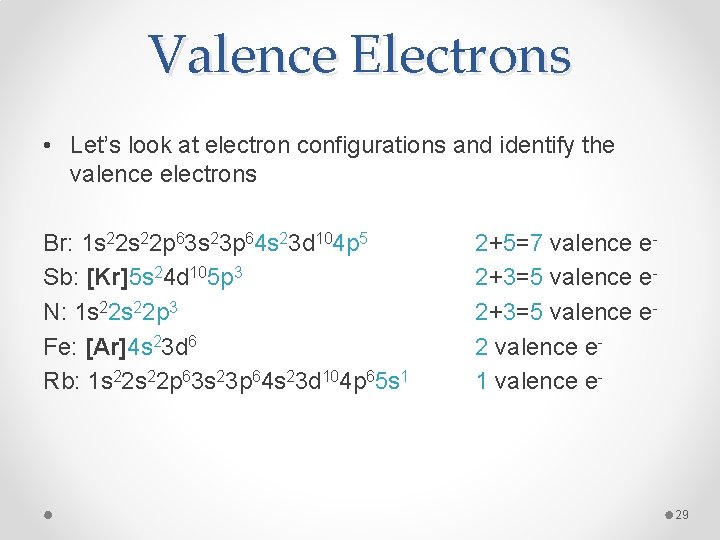
| IA | Li | 1s22s1 | Li+ | 1s2 |
| Na | 1s22s22p63s1 | Na+ | 1s22s22p6 | |
| K | 1s22s22p63s23p64s1 | K+ | 1s22s22p63s23p6 |
The next shell down is now the outermost shell, which is now full — meaning there is very little tendency to gain or lose more electrons. The ion's electron configuration is the same as the nearest noble gas — the ion is said to be isoelectronic with the nearest noble gas. Atoms 'prefer' to have a filled outermost shell because this is more electronically stable.
- The Group IIA and IIIA metals also tend to lose all of their valence electrons to form cations.
| IIA | Be | 1s22s2 | Be2+ | 1s2 |
| Mg | 1s22s22p63s2 | Mg2+ | 1s22s22p6 | |
| IIIA | Al | 1s22s22p63s23p1 | Al3+ | 1s22s22p6 |
- The Group IV and V metals can lose either the electrons from the p subshell, or from both the s and p subshells, thus attaining a pseudo-noble gas configuration.
| IVA | Sn | [Kr]4d105s25p2 | Sn2+ | [Kr]4d105s2 |
| Sn4+ | [Kr]4d10 | |||
| Pb | [Xe]4f145d106s26p2 | Pb2+ | [Xe]4f145d106s2 | |
| Pb4+ | [Xe]4f145d10 | |||
| VA | Bi | [Xe]4f145d106s26p3 | Bi3+ | [Xe]4f145d106s2 |
| Bi5+ | [Xe]4f145d10 |
- The Group IV - VII non-metals gain electrons until their valence shells are full (8 electrons).
| IVA | C | 1s22s22p2 | C4- | 1s22s22p6 |
| VA | N | 1s22s22p3 | N3- | 1s22s22p6 |
| VIA | O | 1s22s22p4 | O2- | 1s22s22p6 |
| VIIA | F | 1s22s22p5 | F- | 1s22s22p6 |
Kr Valence Electrons
- The Group VIII noble gases already possess a full outer shell, so they have no tendency to form ions.
- Transition metals (B-group) usually form +2 charges from losing the valence s electrons, but can also lose electrons from the highest d level to form other charges.
| B-group | Fe | 1s22s22p63s23p63d64s2 | Fe2+ | 1s22s22p63s23p63d6 |
| Fe3+ | 1s22s22p63s23p63d5 |
References
Martin S. Silberberg, Chemistry: The Molecular Nature of Matter and Change, 2nd ed. Boston: McGraw-Hill, 2000, p. 277-284, 293-307.
Condensed Electron Configuration Of Krypton
Kr Valence Electrons Vs
